A periodic table (also known as the periodic table of elements) is arranged in such a manner so that you can easily distinguish the properties of various elements, such as their mass, electron number, electron structure, and their unique chemical compositions. It is a display of chemical elements organized by atomic number, electron configuration, and chemical properties. When you go through this table, you will see that metals and nonmetals are on different sides of the table. The metals appear on the left, while non-metals appear on the right side. This table is also used in schools in chemistry lessons to help students understand the various elements, their properties, and interactions with each other. For you to understand this table comprehensively, knowing what the periodic law states are essential.
It states that when elements are organized in order of increasing atomic mass, some groups of properties recur regularly. This law was established by Dmitri Mendeleev and Lothar Meyer in 1869. Periodic law is considered to be among the most fundamental principles of chemistry. All chemists use the Periodic Law when working with chemical elements, their properties, and chemical reactions. Periodic law has contributed to the creation of the modern periodic table.
In this article, we have explained the periodic table in its fullness to help you understand it better than before.
Free Charts
The periodic table is a scientific table consisting of rows and columns. Columns are the groups, while rows are the periods. The rows and columns are then packed with elements corresponding to the different groups and periods indicated. You need to know the number of electrons an atom has to understand this table. Here, we have periodic table charts that are easy to understand. Feel free to download them and gain knowledge and understanding of this table.
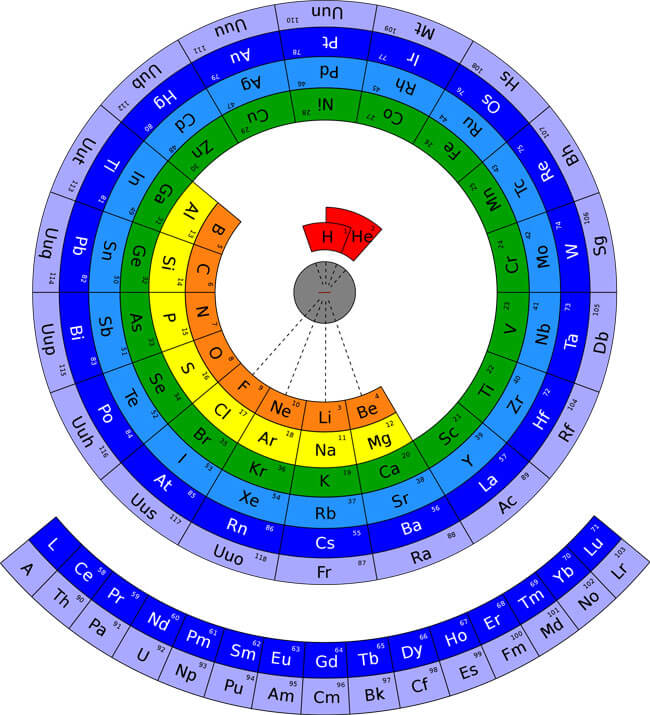
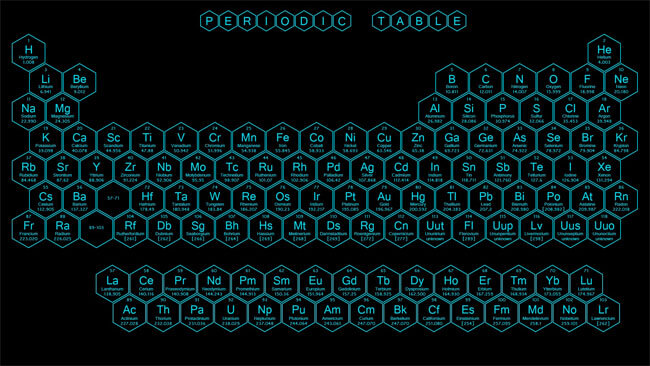
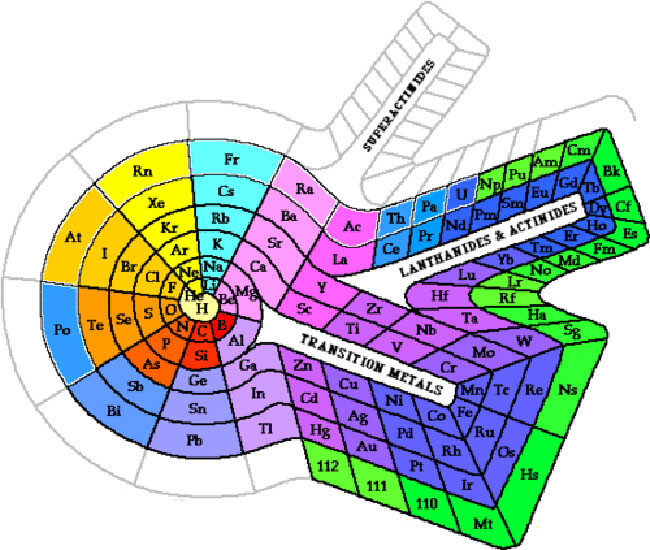
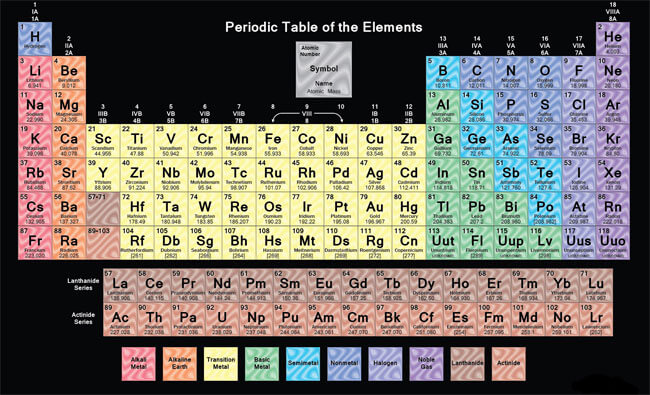


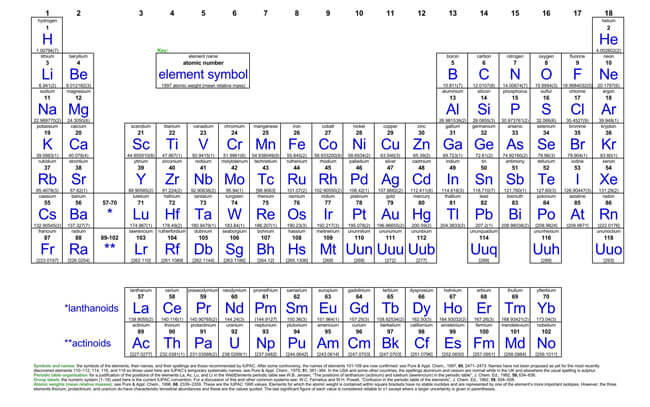
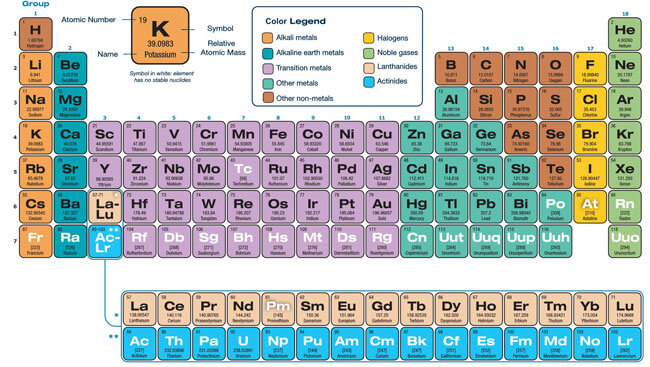
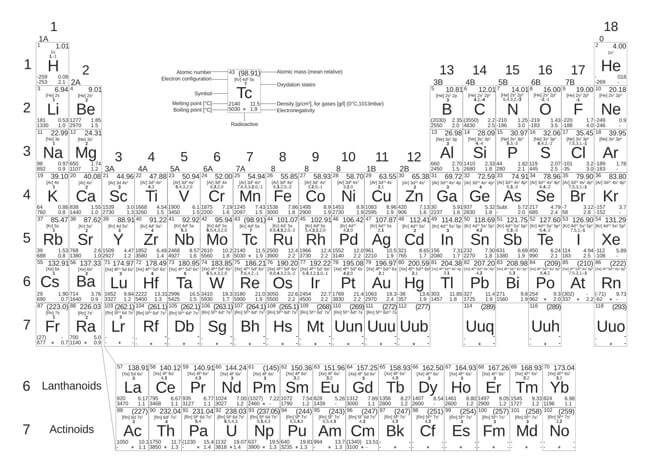
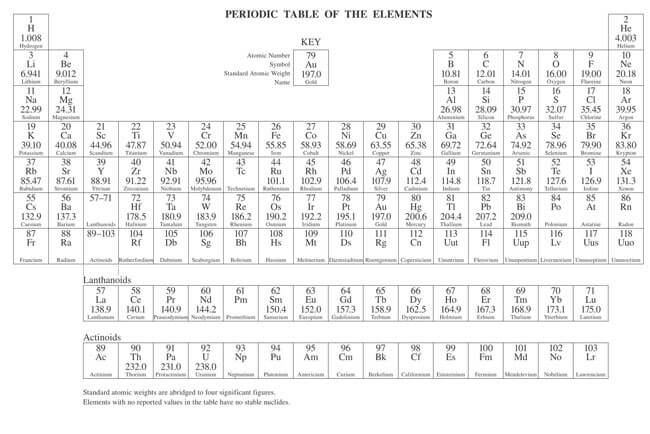
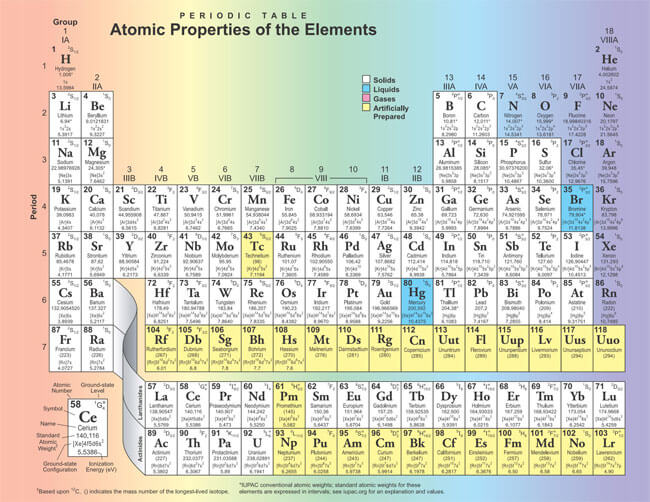

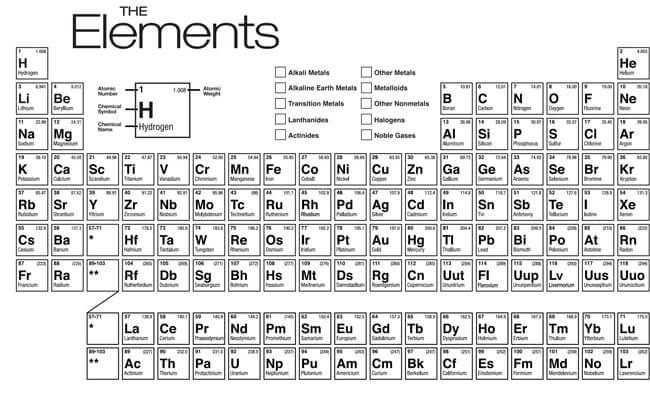

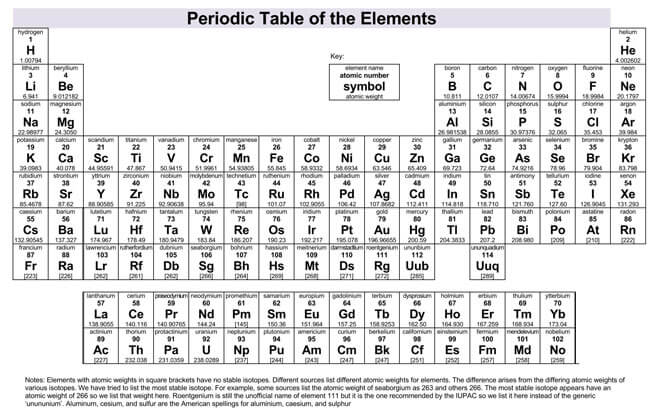
Modern Periodic Table
This table is also used to group the elements today. The modern table is based on Mendeleev’s table, except that the modern table arranges the elements by increasing the atomic number instead of the atomic mass. The atomic number is the number of protons in an atom, which is unique to each element. The new table has more elements than Mendeleev’s table since more elements have been found since Mendeleev’s time.
Who Invented It
The elements of the periodic table were not all discovered at the same time. Different scientists found various elements at different times.
The following people are the ones who found different elements in the periodic table:
John newlands
Around47 elements were identified at the beginning of the19th century, giving enough evidence for chemists to begin to see trends. John Newlands wrote his Rule of Octaves in 1865. The Rule of Octaves had two elements in one box and did not make space for undiscovered elements, so it was ridiculed and did not gain attention.
Lothar meyer
A year earlier (1864), Lothar Meyer released a periodic table describing the placement of 28 elements. Meyer’s table divided the elements into groups organized according to their atomic weights. His table classified the elements into six families according to their importance and was the first attempt to group the elements according to this property.
Dmitri mendeleev
Many people assume that Mendelev was the one who invented the modern periodic table. In a lecture to the Russian Chemical Society, Dmitry Mendeleev introduced his periodic table of elements based on increasing atomic weight on 6 March 1869. Although Mendeleev’s table was the first to achieve recognition in the science world, it was not the first table of its kind. Some elements, such as Gold, sulphur, and carbon, have been known since ancient times. Alchemists started to explore and recognize new features in the 17th century.
Although Mendeleev’s table was almost there, it needed a significant improvement before it became a modern periodic table (using the atomic number as the guiding concept for periods). According to Henry, identical properties recur regularly when the elements are organized by increasing atomic number. Atomic quantities, not weights, determine the chemical properties component. The use of the atomic number as the grouping principle was first suggested by the British chemist Henry Moseley in 1913.
Structure of the Periodic Table
The periodic table has various structures that make it complete. We have explained each structure below for you to understand the periodic table well.
The structures are:
Periods
he rows on the periodic table are called periods.All the elements in a period have the same number of atomic orbitals.
EXAMPLE
Each element in the top row (the first period) has an orbital for its electrons. All the elements in the second row have two orbitals for their electrons. As you move down the table, an orbital is attached to each row. There are no more than seven electron orbitals at this time. (An orbital is a region where an electron resides in an atom).
The diagram below shows period 2 of the periodic table:
| 3 Li | 4 Be | 5 B | 6 C | 7 N | 8 O | 9 F | 10 Ne |
| Lithium | Beryllium | Boron | Carbon | Nitrogen | Oxygen | Fluorine | Neon |
Groups
The periodic table also has a particular name for its vertical columns. Each column is called a group. The elements of each group have the same number of electrons in the outer orbit. These outer electrons are also called valence electrons. They are electrons involved in chemical bonding with other elements. Each element in the first column (group one) has one electron in its outer shell. Each element in the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you’ll know how many electrons there are in the outer shell.
note
There are exceptions to the order when looking at the transition elements.
The diagram below shows elements in group 2:
| 4 Be Beryllium |
| 12 Mg Magnesium |
| 20 Ca Calcium |
| 38 Sr Strontium |
| 56 Ba Barium |
| 88 Ra Radium |
Blocks
Specific parts of this table can be referred to as blocks considering the order in which the electron shells of the elements are filled. Elements are allocated to blocks in which orbitals their valence electrons or vacancies lie in. The s-block consists of the first two classes (alkali metals and alkaline earth metals) and hydrogen and helium. The p-block has the last six groups, which are 13 to 18 in the IUPAC group numbering (3A to 8A in the American group numbering) and includes, among other elements, all metalloids. The d-block consists of groups 3 to 12 (or 3B to 2B in American group numbering) and includes all transition metals and the f-block comprises the Lanthanide and Actinide series.
Classes
The periodic table elements are grouped into nine categories; six for metals and two for non-metals, and one for metalloids. Different writers can use various categorization schemes based on the properties of interest. Or you can put them in three classes; metals, non-metals, and metalloids.
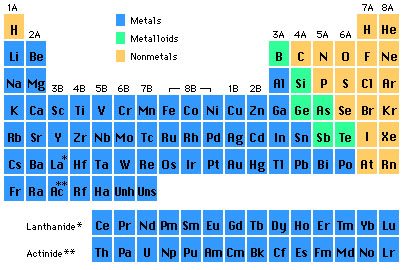
Image source: Prof. N. De Leon
Information Given In the Periodic Table
Chemistry is not limited to activities that happen in the laboratory. Everything on this planet is made up of atoms – the same elements seen on this table. Your first experience with the periodic table may be quite intimidating at first. In reality, most students find it quite confusing. With such a vast volume of information presented, students sometimes miss and mess up all the information on the table. However, with a clearer knowledge of the details provided to you in this table, it can be easier to tackle chemistry issues.
The following information is present on the periodic table:
Atomic number
First is the atomic number. The atomic number of the element is referred to as the number of protons in an atom. The number of protons describes what the element is and also specifies the chemical behavior of the element.
Atomic weight
There is also the atomic weight/mass. The basic atomic weight of an element is the total mass of the element in the atomic mass unit. Specific atoms often have an integer number of atomic mass units; moreover, the atomic mass on the periodic table is indicated as a decimal number. It is the average of the different isotopes of the element. To get the average number of neutrons in an element, you subtract the atomic number from the atomic mass.
Atomic symbol
There is also the atomic symbol. An atomic symbol is an abbreviation that represents an element.
EXAMPLE
H for hydrogen. These symbols are used globally.
Periodic Trends
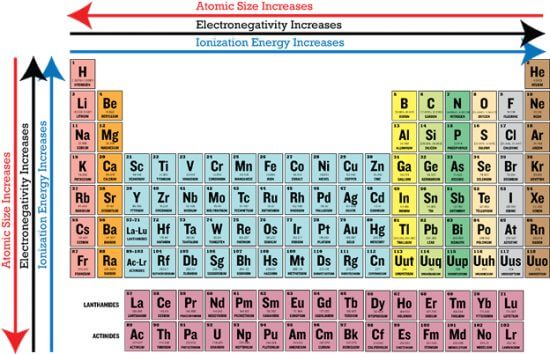
Source of image: CK-12 Foundation
Periodic patterns are based on the Periodic Law, which specifies that when chemical elements are listed according to increasing atomic number, all of their properties undergo gradual changes, with elements with identical properties repeating at intervals.
We have explained the periodic trends for you in the paragraphs below:
Atomic radius
Atomic and ion radius are a measure of the size of a single atom or ion. While the atomic and ion radius is different from each other, they adopt the same general pattern.
Atomic radius is the atomic nucleus’s distance to the outermost stable orbital electron of an atom at equilibrium. Atomic radius grows when you go down a group due to the introduction of a new energy level. Covalent radius, Van der Waals radius, metallic radius, and ionic radius are the four types of atomic radius.
Ionization energy
Ionization energy is used to detach electrons from gaseous atoms or ions. It is different for electrons with different atomic or molecular orbits.
The ionization energy continues to increase as you go from left to right across a period or up a group in a table.
Electron affinity
Electron affinity is measured only for atoms and molecules in the gaseous state. Interactions with other atoms or molecules can modify their energy levels in solid or liquid states.
A molecule or atom with a more positive electron affinity value is called an electron acceptor; one with a less positive electron affinity is called an electron donor. The electron affinity of the elements usually increases across a period and often decreases down the group in the table.
Electronegativity
Electronegativity is the tendency of an atom to attract the shared pair of the electrons between the two atoms.
Trend-wise, when you go from left to right across a period in the table, electronegativity increases due to the greater attraction that the atoms receive as the nuclear charge increases. As you go down a group, electronegativity decreases due to an increase in the space between the nucleus and the valence electron layer, which lowers the attraction, making the atom less attractive to electrons or protons.
Metal character
The metallic character indicates the reactivity of a metal.
Metal character increases as you go down the groups. The decreasing attraction between the nucleus and the outermost electrons allows the outer electrons to be loosely bound and conduct heat and electricity. The increasing attraction between the nucleus and the outermost electrons decreases the nucleus’s metallic quality from left to right across the period.
Common Family Names
The word “family” is used to describe elements that share similar characteristics—not only in terms of visible behavior but also in terms of atomic structure.
Below is a periodic table showing the common family names:
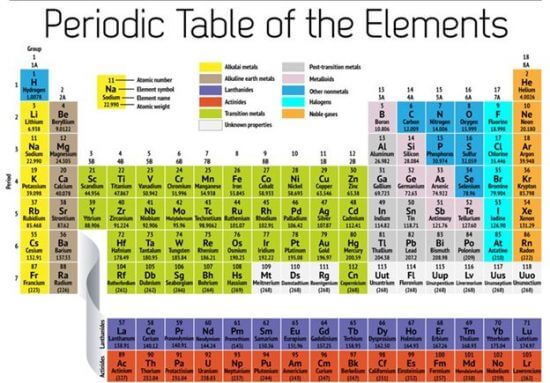
Image source: NBC news
Frequently Asked Questions
The discovery of individual elements was a required condition for the creation of a periodic table. While gold, silver, tin, copper, lead, and mercury have been known since ancient times, the first element discovered was phosphorous in 1649 by Hennig Brand.
With a boiling point of 3,422 degrees Celsius/6,192 degrees Fahrenheit, Tungsten has the highest melting point of all metals.
‘Au’ is derived from the word ‘Aurum’ even though the word gold is Anglo-Saxon.
Dmitri Mendeleev is widely and popularly known as the father of the periodic table.
Conclusion
You can use the periodic table’s organization to establish the relationship between the different properties of the elements and predict the chemical properties and behavior of undiscovered or newly synthesized elements. Russian chemist Dmitry Mendeleev published the first recognizable periodic table in 1869, created primarily to explain the then-known elements’ periodic patterns. He also predicted certain properties of unspecified elements that were supposed to fill the gaps in the table. Most of his predictions quickly proved accurate, resulting in gallium and germanium discoveries in 1875 and 1886, which verified his predictions.




