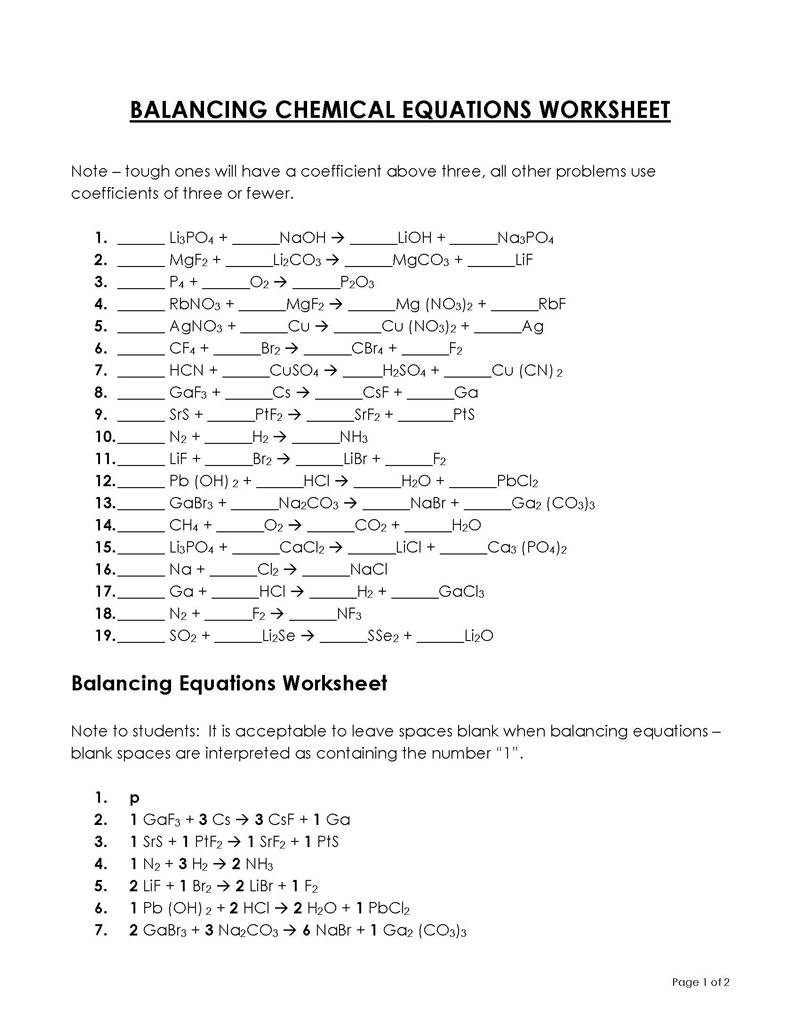
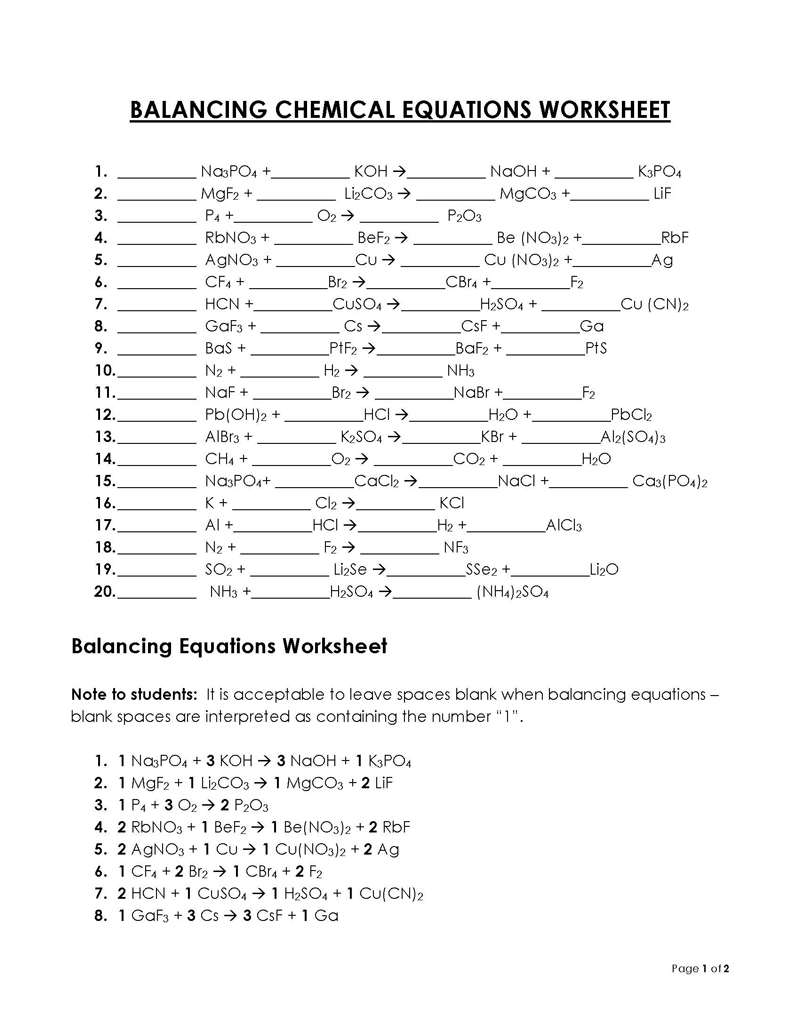
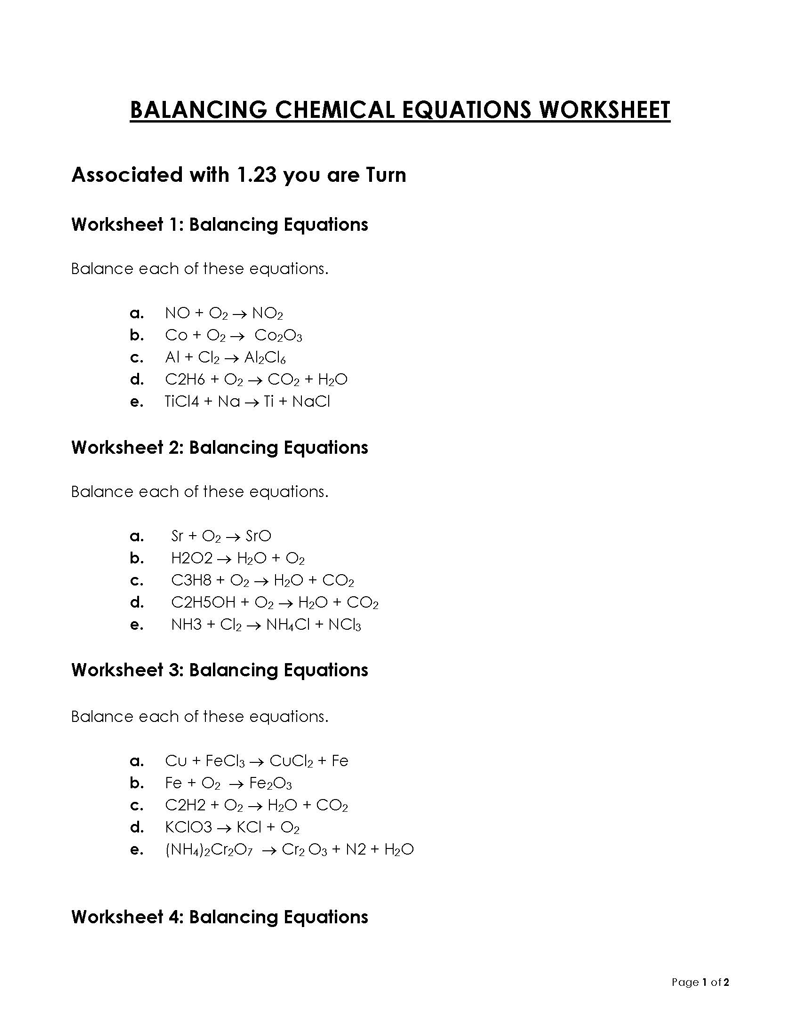
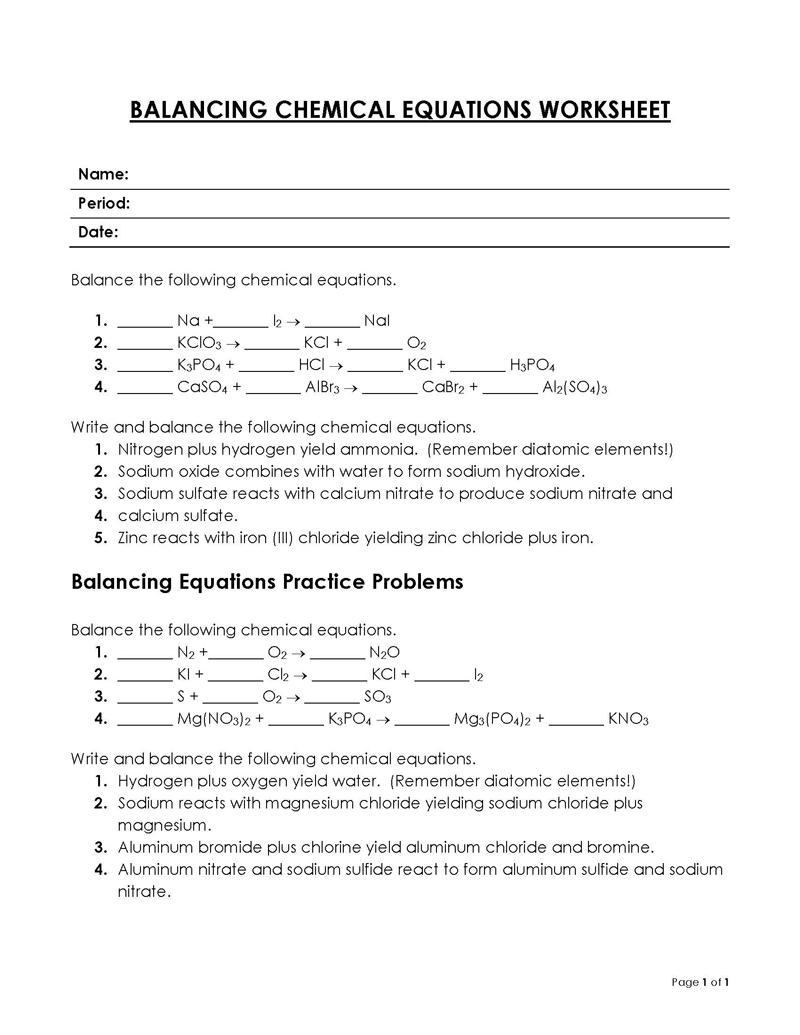
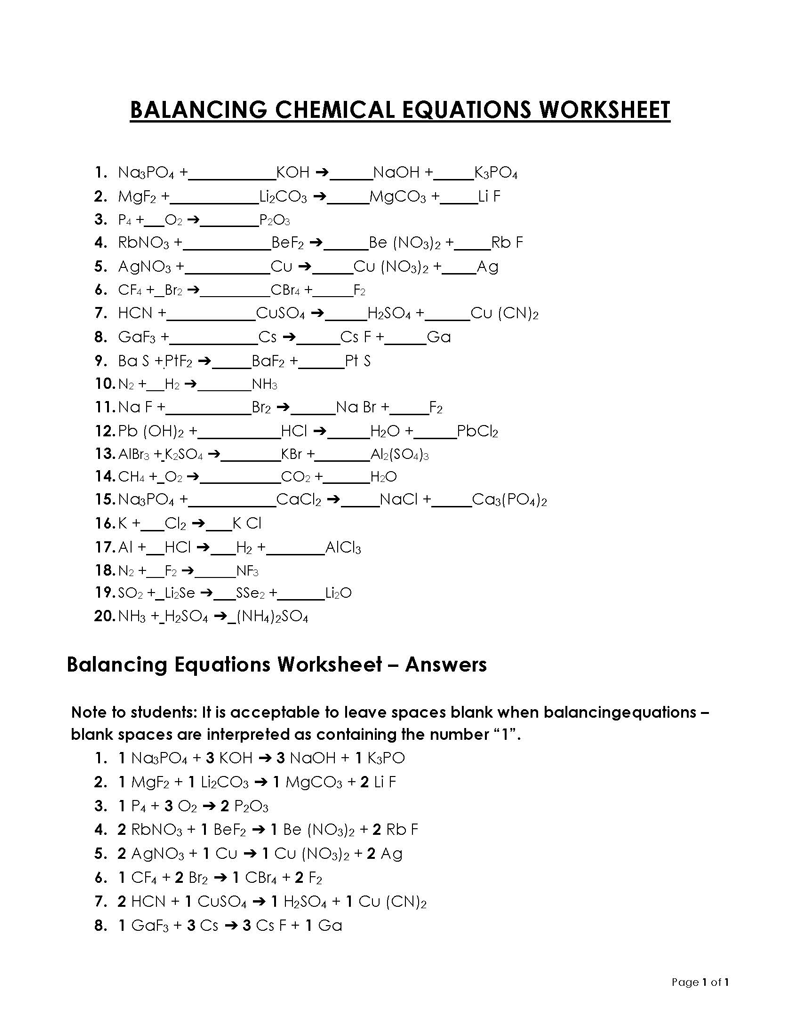
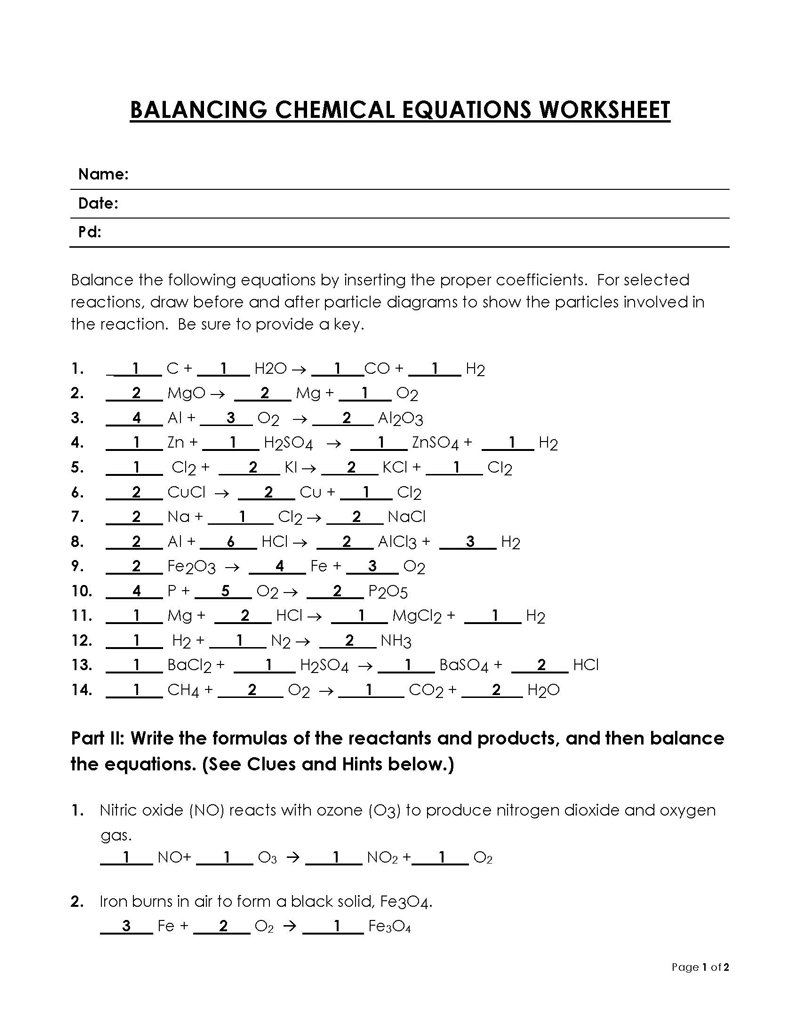
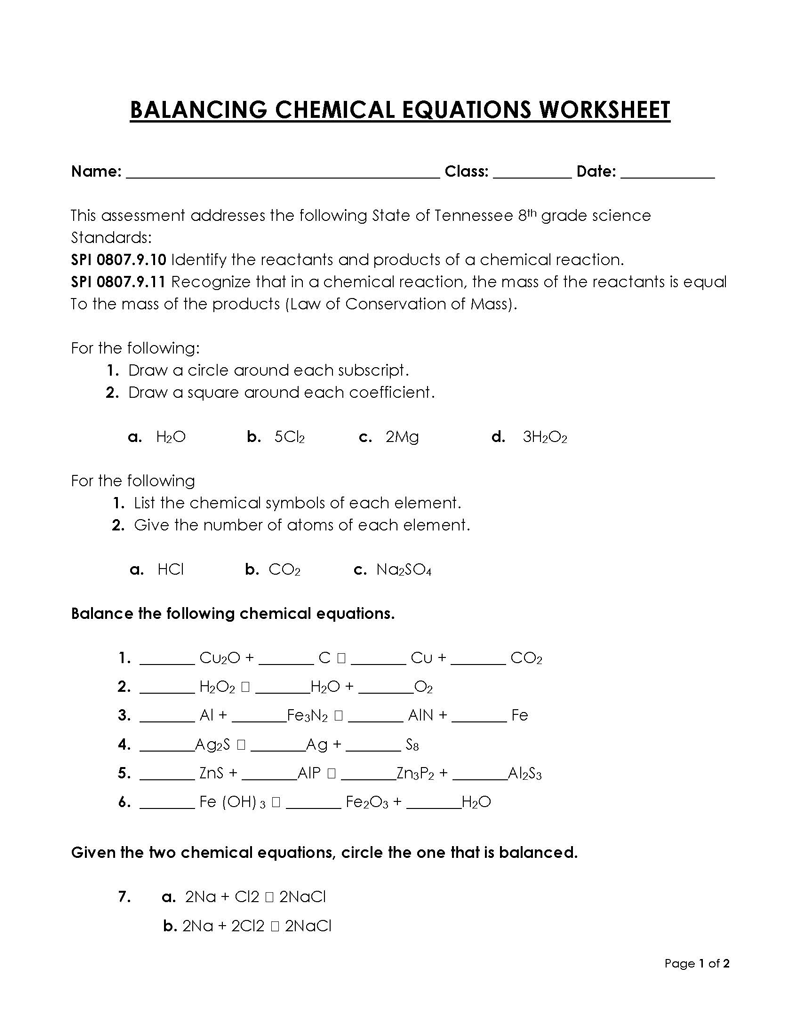
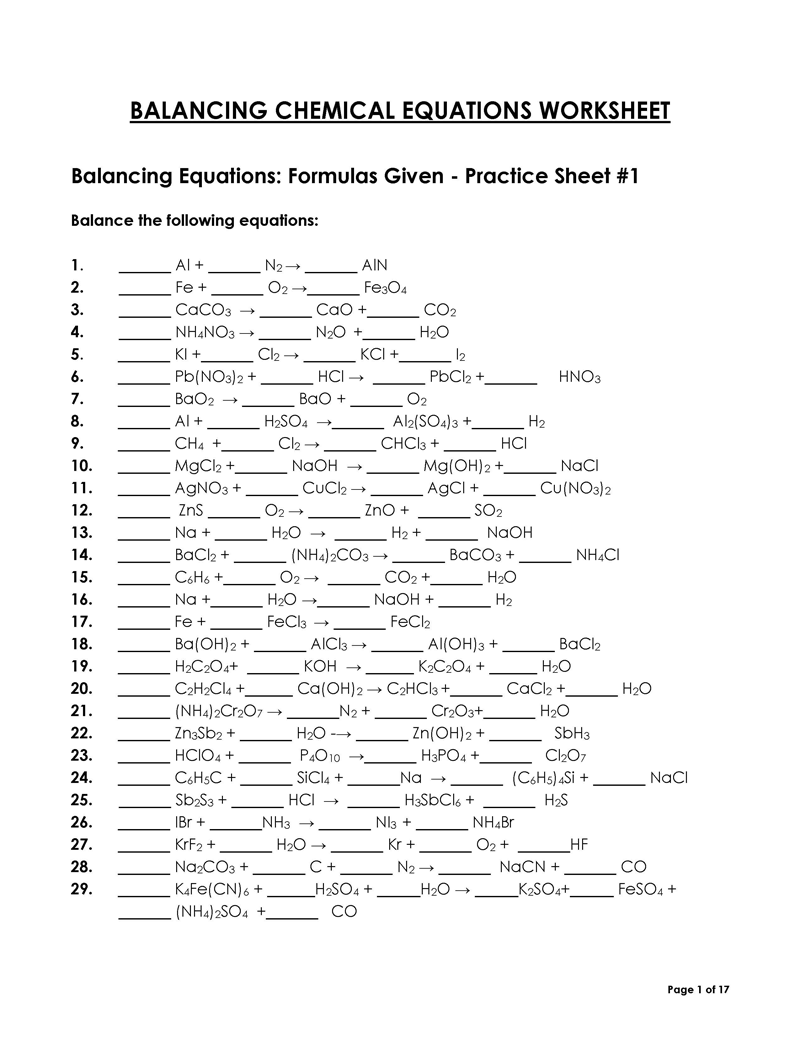
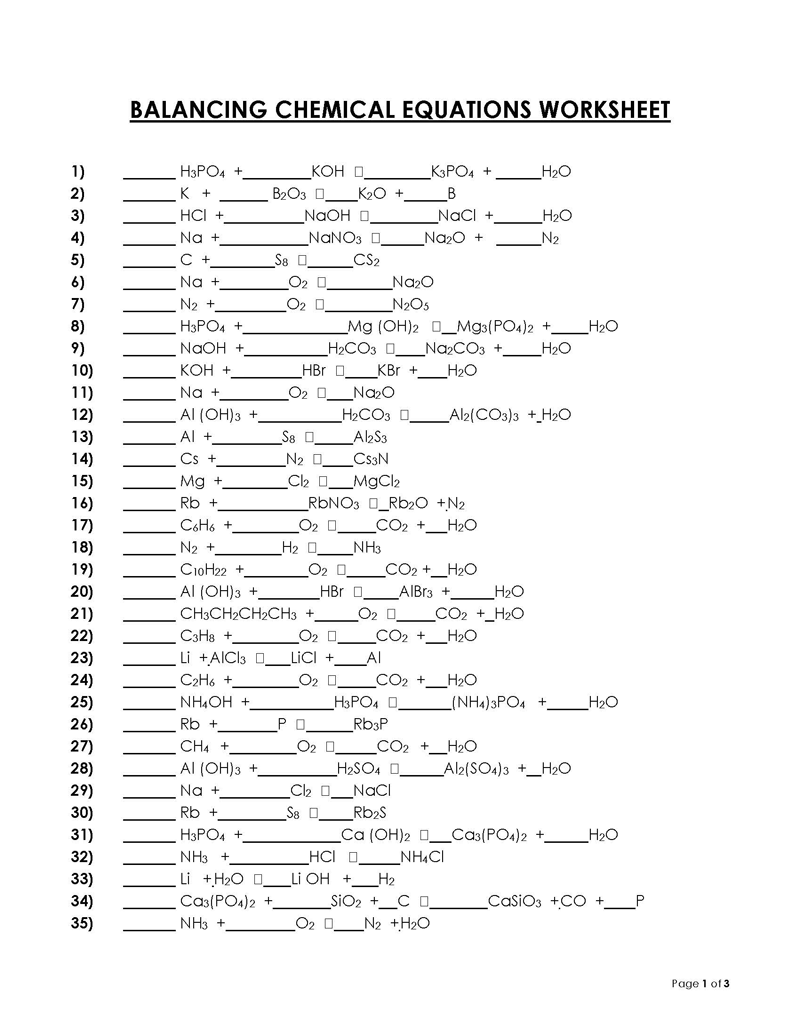
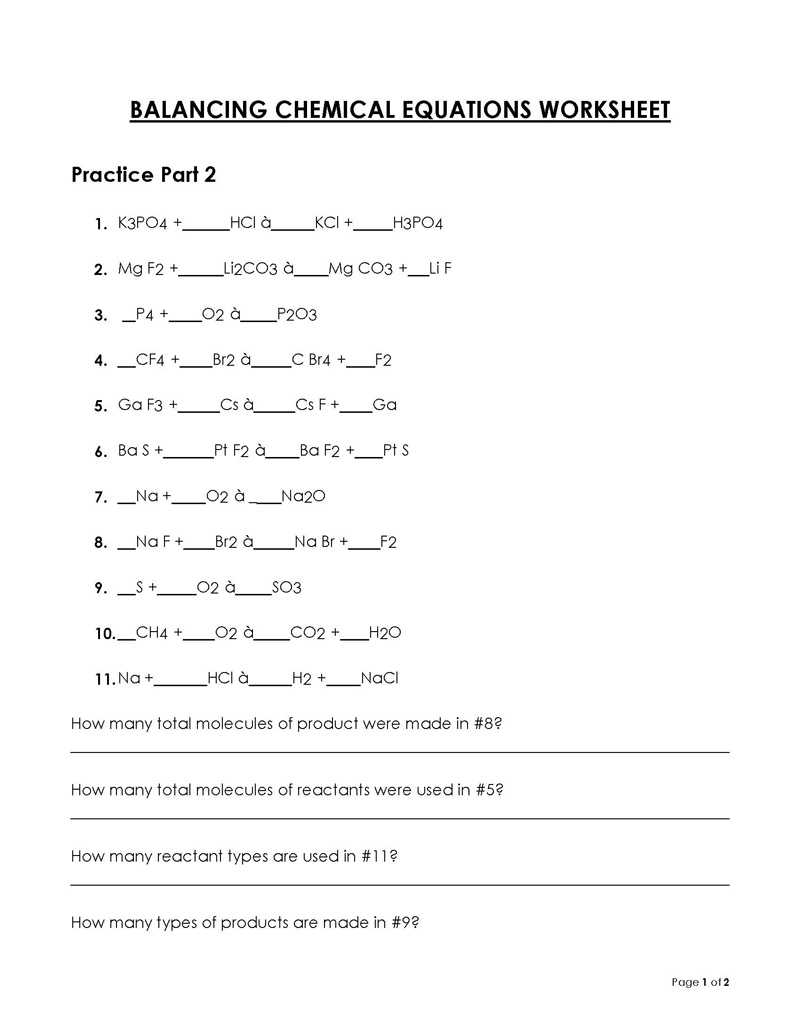
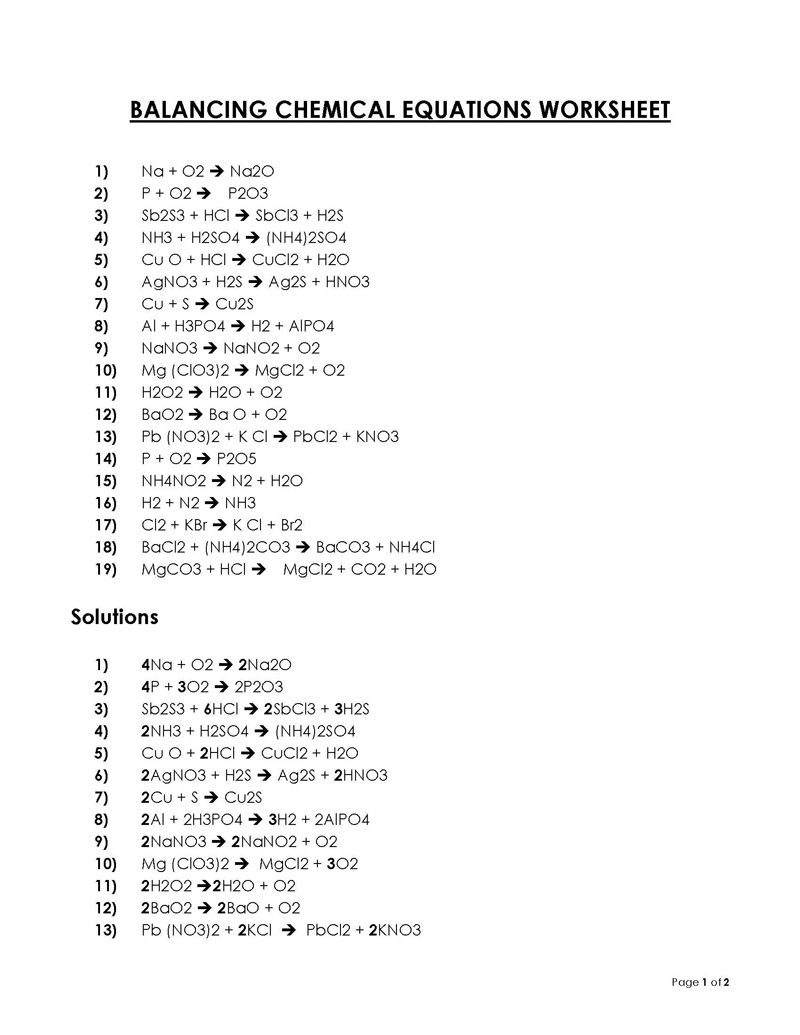
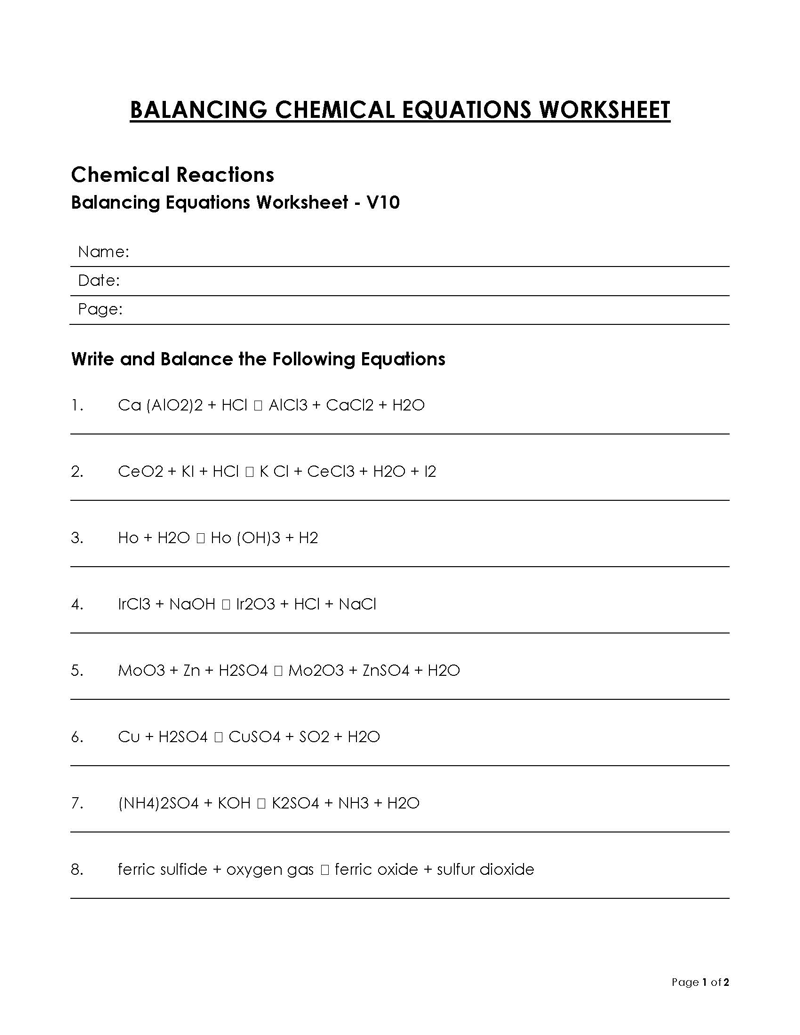
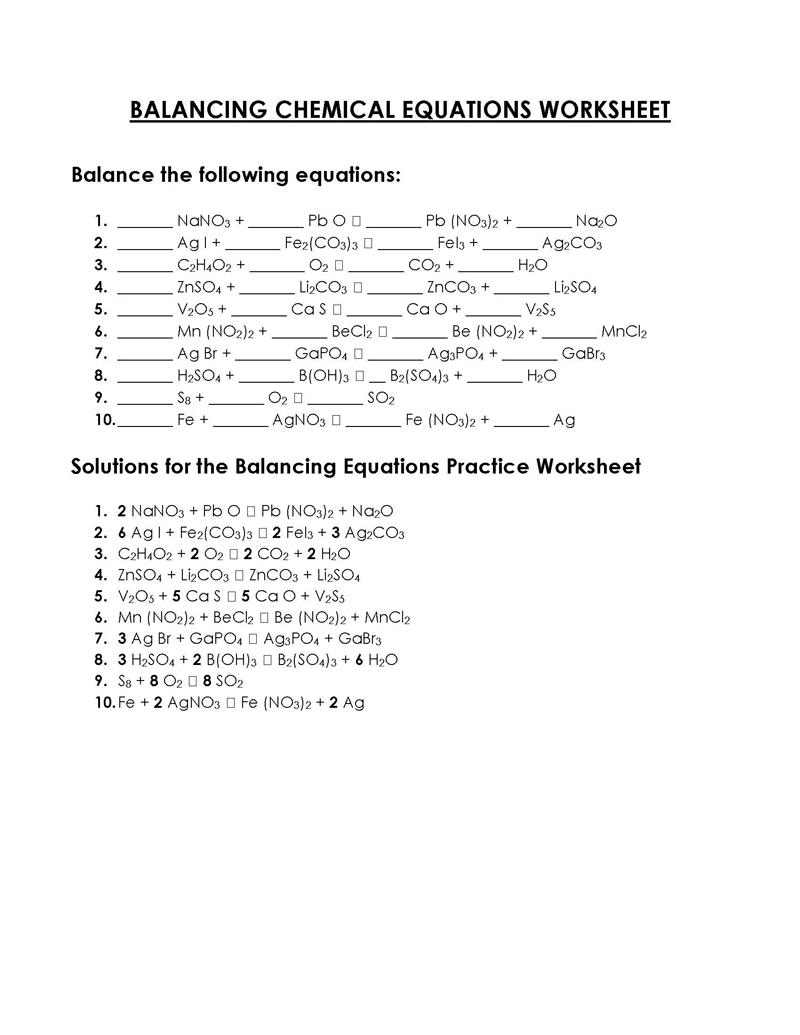
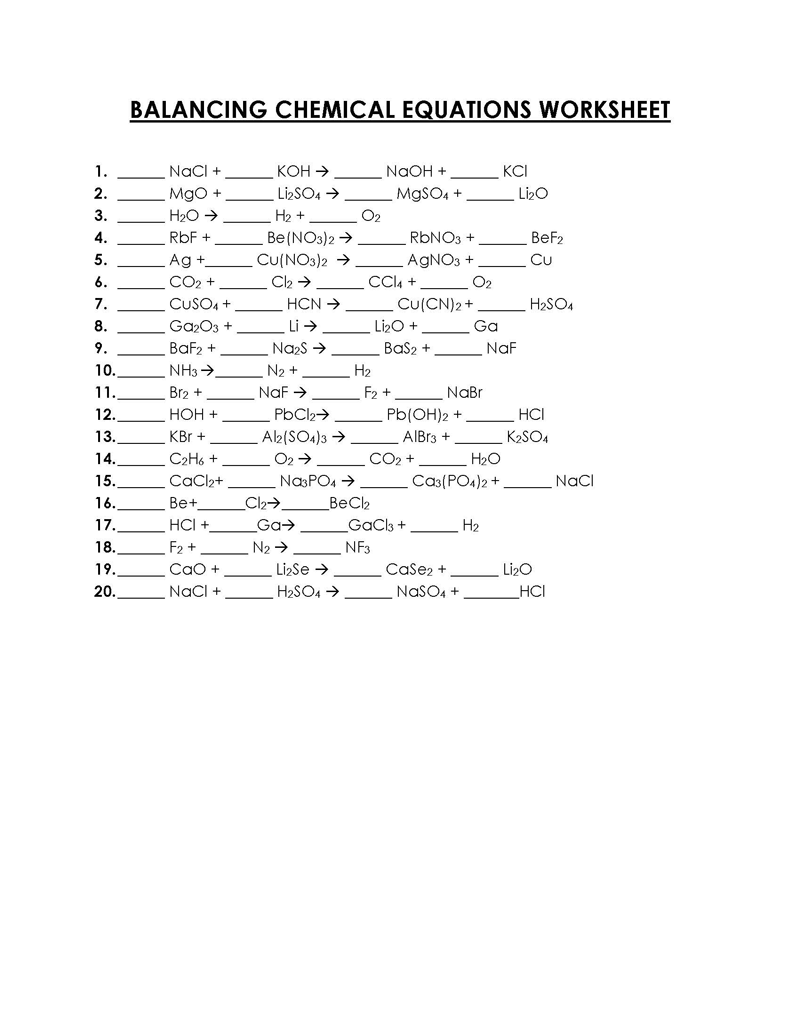
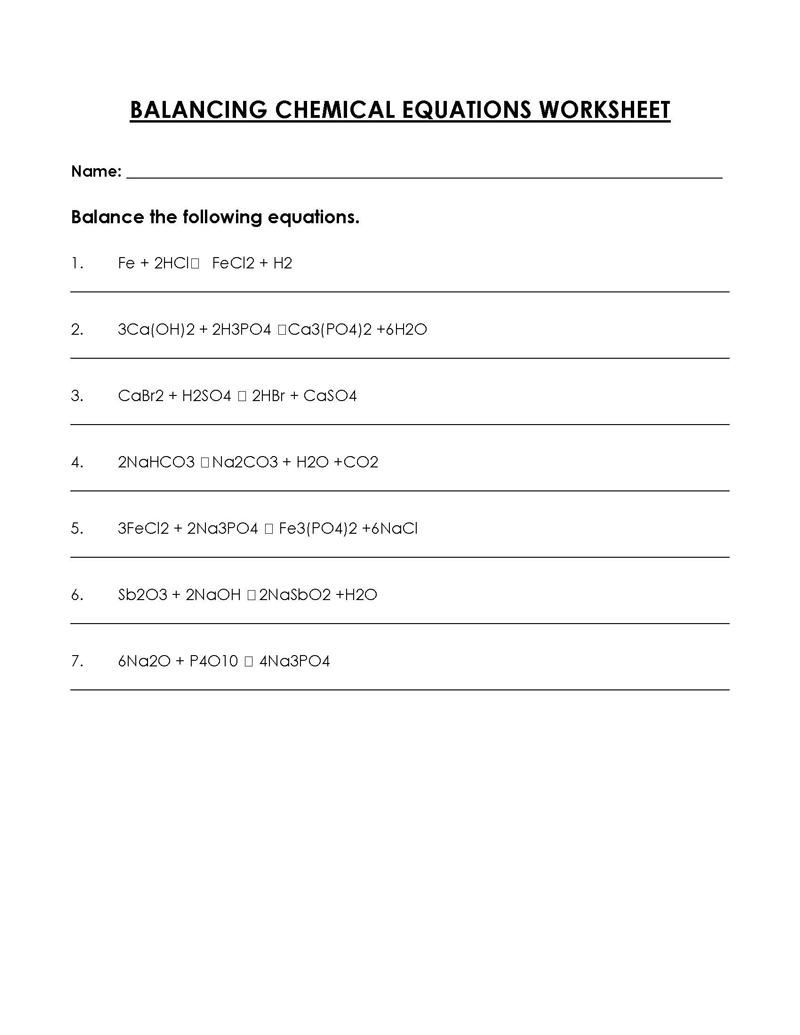
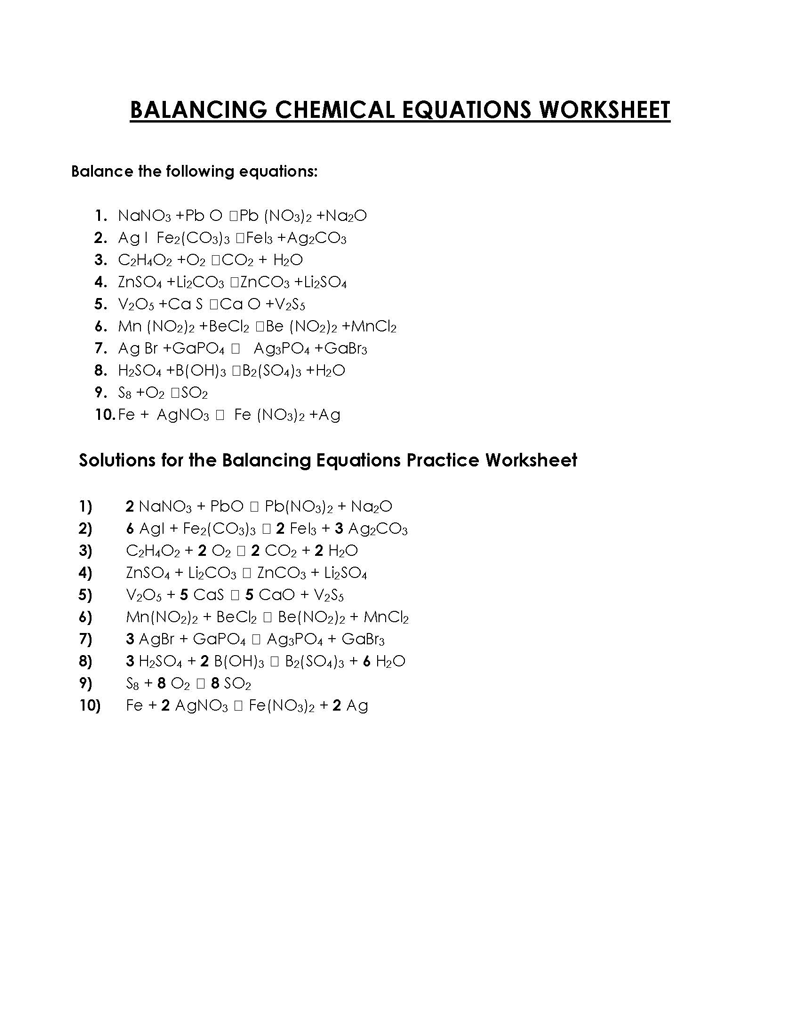
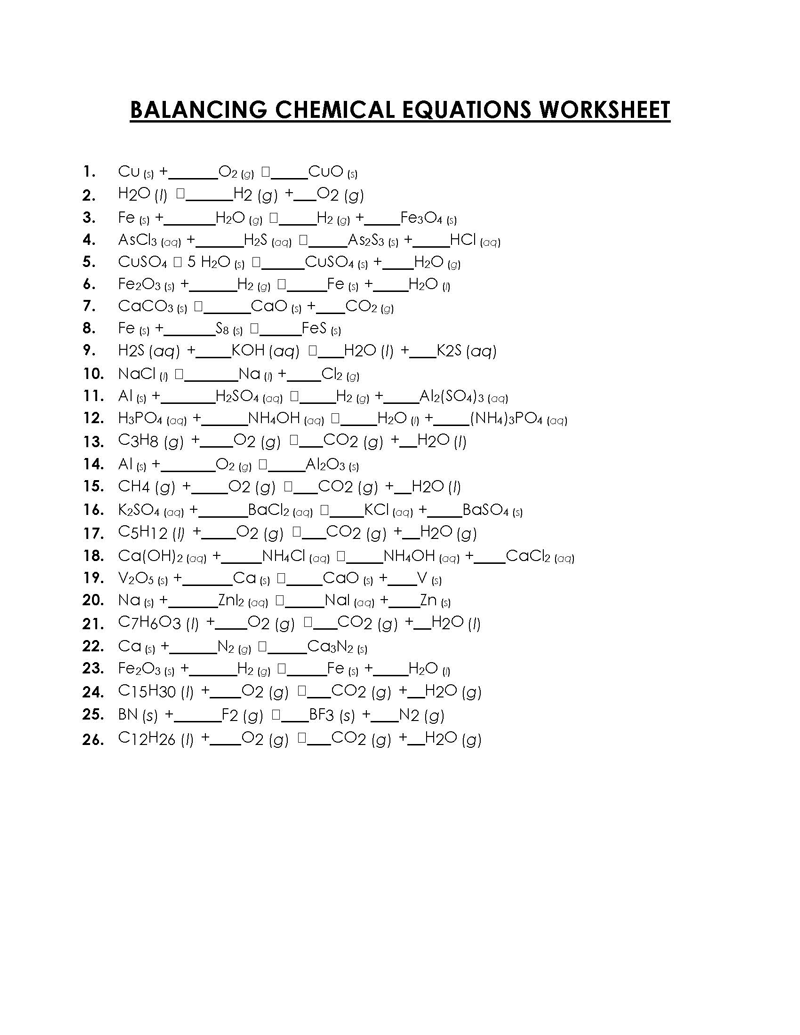
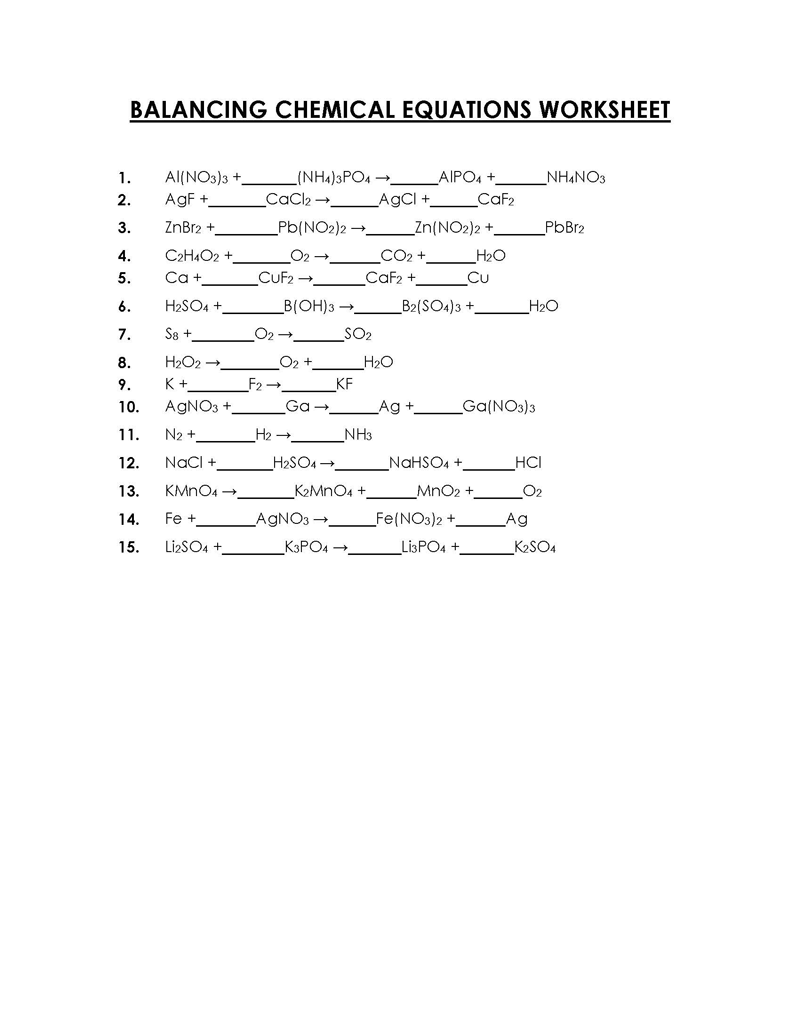
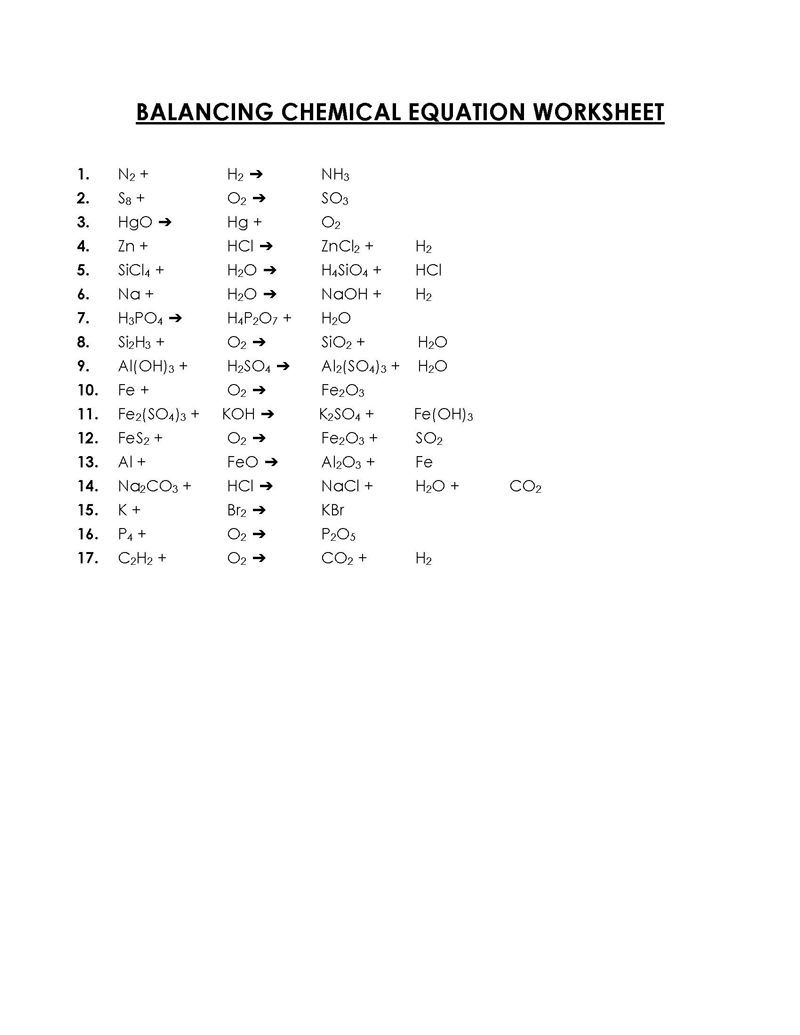
Balancing a Chemical Reaction is the process of evening out the mass (atoms) and charge on both sides (reactants and products) of a chemical reaction/equation.
Balancing chemical equations involves calculations of molecules and atoms in the equation. The number of molecules is represented using coefficients, and subscripts represent the atoms.
A balanced equation ensures the law of conservation of mass – mass or matter can neither be created nor destroyed, just transformed – is held such that atoms (mass) and charge of the reactants equals that of the products. Thus, by interpretation, atoms cannot be created or destroyed. Without satisfying this law, the equation cannot be true – the number of reactants in that equation would not result in the number of products of the same equation. Therefore, the mass of a chemical compound can be accurately determined and preserved.
Since different elements or substances combine to form new products, balancing the chemical equation ensures the same reaction can be replicated for research or manufacturing purposes.
Free Templates
Balanced Vs. Unbalanced Chemical Equation
An unbalanced equation has the mass and/or the charge of reactants unequal to those of the products. Thus, an unbalanced chemical equation does not state the amount needed for the reaction to observe the conservation of mass.
For example:
C2 H4 (g) + O2 (g) —————-> CO2 (g) + H2 O(l)
(g) represents gas, (l) is liquid and (s) is solid.
Why is the above equation unbalanced?
– The number of atoms of each element on the right and the left sides is unequal. There are 2 (two) carbon atoms on the left-hand side (L.H.S) and 1 on the right-hand side (R.H.S).
– There are 4 (four) atoms of hydrogen on the L.H.S and 2 on the R.H.S.
– There are 2 oxygen atoms on the L.H.S and 2 on the R.H.S.
A balanced chemical equation has the mass (atoms) and the charge of the reactants equal to those of the products. Therefore, a balanced chemical equation satisfies the law of conservation of mass.
An example of a balanced equation is as shown below:
C2 H4 (g) + 3O2 (g) ————–> 2CO2 (g) + 2H2 O (l)
It, therefore, requires 1 molecule of methane (C2 H4) and 3 molecules of oxygen gas (3O2) to produce 2 molecules of carbon (II) oxide (2CO2) and 2 molecules of water (2H2 O).
The equation is balanced because there are equal numbers of atoms of each element on both sides of the equation; 2 carbon atoms, 3 hydrogen atoms, and 6 oxygen atoms.
Types of Balanced Chemical Equation
A chemical equation can be molecular or ionic. One aqueous reaction can be written as either of the two types of chemical equations depending on the number of details needed and the application of the equation.
These equations differ in the following ways:
Molecular
A molecular equation represents the reactants and products as neutral substances and does not show the charge of each element or ionic compound. The molecular equation indicates the state of each substance of the reaction; gas, solid or aqueous.
For example:
Mg(s) + 2HCL(g) —————-> MgCl2(aq) + H2(g)
Ionic
Ionic equations indicate the aqueous substances reacting or resulting from a chemical reaction. The equation thus shows the ionic species present in the solution. This is important so as to show that constituent ions of ionic compounds still exist homogenously even after dissolving in water; they are simply dissociated.
For example:
Mg (s) + 2H+ (aq) + 2Cl– (aq) —————-> Mg+2 (aq) + 2Cl– (aq) + H2(g)
Only soluble ions are broken down into their respective constituent ions. The charge and state of each ion are shown in an ionic equation. Coefficients are used to indicate the number of each ion in the solution, and subscripts are not used. Most covalent compounds (gases and solids) and elements are not broken down into ionic equations as they are insoluble in water.
Ionic compounds can be further categorized into either complete ionic or net ionic equations depending on the inclusion of spectator ions. Spectator ions are ions that are present in the solution but do not participate in the precipitation reaction; they are neither physically nor chemically changed by the reaction. The purpose of spectator ions is to preserve the equation’s charge neutrality.
The difference between a complete ionic and net ionic equation is as discussed below:
- Complete ionic: A complete ionic chemical equation indicates all the dissolved ions in the reaction.
Below is an example of a complete ionic equation:
Mg (s) + 2H+ (aq) + 2Cl– (aq) —————> Mg+2 (aq) + 2Cl– (aq) + H2(g)
- Net ionic: A net ionic chemical equation eliminates the spectator ions such that only the ions actively involved in the reaction are given.
An example of a net ionic equation is given below:
Mg(s) + 2 H+(aq) —————–> Mg+2(aq) + H2(g)
Fundamental Aspects of a Balanced Chemical Equation
To understand how to balance chemical equations accurately, some items should first be considered.
These are:
- Reactants: A reactant is any substance present at the start of a chemical reaction and is expected to undergo a physical or chemical change by the end of the reaction. Reactants are placed on the L.H.S.
- Products: A product is any substance that results from a chemical reaction or a physical change. Products are written on the R.H.S of a chemical equation.
- Coefficients: A coefficient is a number used to show the relative amount in a chemical equation. Coefficients are placed in front of symbols or formulas in the equation.
- Physical state: A substance can either be solid, liquid, gas or aqueous (dissolved in water). The proper notations must be used to describe each substance in a chemical equation.
Note: Some reactions only take place under certain conditions. The applicable special conditions of any chemical equation must be indicated. It can be written as a word or symbol below or above the equation arrow.
How to Balance a Chemical Equation
Balancing chemical equations can be complex or straightforward, depending on the type of reaction and the complexity of participating substances. The process involves altering the coefficients of the equation, NOT the subscripts. Subscripts represent the identity of the substances or elements. A trial and error or “inspection” method are used to balance a chemical reaction.
Below are the steps used to carry out the process:
Step 1
Identify the most complex compound or substance. Break it down into the different constituting element(s).
Eq 1: C3H8 + O2 → CO2 + H2O
Eq 2: FeCl3 + NaOH → NaCl + Fe(OH)3
Eq 3: Ca(OH)2 + HNO3 → Ca(NO3)2 + H2O
Step 2
Adjust the coefficients. Identify the element(s) that appear the least number of times in the equation; preferable in one reactant and one product. If a number has not to be indicated in front of a substance, always take the number as 1. Ensure the same number of atoms of the selected element appear on both L.H.S and the R.H.S.
Eq 1: C3H8 + O2 → 3CO2 + 4H2O
Carbon and hydrogen are the elements that appear least times. Starting with carbon, there are 3 carbon atoms in LHS and 1 in the RHS, therefore to balance carbon atoms, input 3 at 3CO2, and the carbons are balanced. 8 hydrogen atoms are balanced by inserting 4 in the RHS, and hydrogen is balanced.
Eq 2: FeCl3 + NaOH → 3NaCl + Fe(OH)3
Balance chlorine by inserting 3 in NaCl, and chlorine is balanced.
Eq 3: Ca(OH)2 + 2HNO3 → Ca(NO3)2 + H2O
Calcium is balanced, so proceed to hydrogen. Insert 2 at HNO3, and hydrogen is balanced.
Step 3
Balance the polyatomic atoms as a unit if they exist on both sides of the equation.
Eq 1: C3H8 + 5O2 → 3CO2 + 4H2O
This equation has no polyatomic atoms.
Eq 2: FeCl3 + 3NaOH → 3NaCl + Fe(OH)3
OH is a polyatomic atom, balanced by inserting 3 at NaOH, and the polyatomic atom is balanced. Adding 3 also balances the sodium (Na) atoms such that there are 3 on both sides. This equation is fully balanced at this point.
Eq 3: Ca(OH)2 + 2HNO3 → Ca(NO3)2 + H2O
NO3 is a polyatomic atom; it was balanced when hydrogen was being balanced. However, note that oxygen is still not balanced. OH is also a polyatomic atom, but its constituents can only be balanced independently since it does not appear on both sides.
Step 4
Balance the other atoms. Preferably, end with the least complex substance. Fractional coefficients can be used if necessary. In that case, multiply both sides by the denominator to remove the fraction. Coefficients should be whole numbers.
Eq 1: C3H8 + O2 → 3CO2 + 4H2O
Balance the oxygen as it is the only remaining atom; there are 10 atoms on the RHS; therefore, add 5 to oxygen such that there are 10 atoms on each side.
Eq 2: FeCl3 + 3NaOH → 3NaCl + Fe(OH)3
This equation is balanced.
Eq 3: Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
Balance oxygen and hydrogen. There are 4 hydrogen atoms on the LHS and 2 on the RHS; insert 2 to balance. Consequently, oxygen gets balanced such that there are 8 oxygen atoms on either side.
Step 5
Calculate the number of atoms of each element on both sides of the equation and ascertain that they are equal. If so, the equation is balanced.
Final Thoughts
Balancing chemical equations is an essential process for anyone in the field of chemistry, a student, or a chemist/practitioner. Balancing chemical equations is essential for conserving the law of conversion of mass between the reactants and the products.
A balancing chemical equations worksheet can be used to check how accurately an equation has been balanced. A chemical equation can be presented as a molecular or ionic equation depending on the amount of detail required.